Chapter 4 - Materials: Metals and Non-Metals Notes
1. Occurrence of metals and non-metals
• Out of the 92 naturally occurring elements 70 are metals and about 22 are nonmetals. Some elements show properties of both metals and non-metals. They are called metalloids.
• Only some metals like gold, silver, platinum etc are found in the free-state. Most metals are found in the combined states as oxides, sulphides, carbonates, silicates etc.
• Some non-metals are found in the free state like helium, neon, argon etc. and some are found in free and combined states like sulphur, phosphorus etc.
2. Metallurgy
• Metallurgy is science of extraction of metals from their ores and their purification.
• Minerals are naturally occurring substances containing one or more elements or their compounds.
• Ore is a mineral from which one or more metals can be extracted profitably.
• Metallurgical processes consists of three main steps. They are:
→ Concentration of the ore: It is the removal of impurities from the ore.
→ Reduction: It is the process of obtaining the metal from its compound.
→ Refining: It is the process of purification of the impure metals to obtain the pure metal.
3. Physical properties of metals and non-metals
• Metals
→ Metals are solid (except mercury).
→ Metals are hard (except sodium, potassium etc).
→ Metals have metallic lustre.
→ Metals have high melting points and boiling points.
→ Metals are malleable (can be made into thin sheets).
→ Metals are ductile (can be made into thin wires).
→ Metals are good conductors of heat and electricity.
→ Metals are sonorous (produces sound).
• Non-metals
→ Non-metals may be solid, liquid or gas.
→ Non-metals which are solid are brittle (diamond is the hardest).
→ Non-metals do not have lustre, some have a dull lustre.
→ Non-metals have low melting points.
→ Non-metals are not malleable.
→ Non-metals are not ductile.
→ Non-metals are bad conductors of heat and electricity (except graphite).
→ Non-metals are not sonorous.
4. Chemical properties of metals and non-metals
• Reaction with Oxygen
Metals react with oxygen to form metallic oxides. These oxides are basic oxides because they react with water to form bases. Example: Magnesium burns in air to form magnesium oxide. Magnesium reacts with water to form magnesium hydroxide.
2Mg + O₂ → 2MgO
MgO + H₂O → Mg(OH)₂
Non-metals react with oxygen to form non-metallic oxides. These oxides are acidic oxides because they react with water to form acids. Example: Sulphur burns in air to form sulphur dioxide. Sulphur dioxide reacts with water to form sulphurous acid.
S + O₂ → SO₂
SO₂ + H₂O → H₂SO₃
• Reaction with Water
Metals react with water to form metal hydroxides and hydrogen. Example: Sodium reacts with water to form sodium hydroxide and hydrogen.
2Na + 2H₂O → 2NaOH + H₂
Magnesium reacts with water to form magnesium hydroxide and hydrogen.
Mg + H₂O → Mg(OH)₂ + H₂
Non-metals do not react with water.
• Reaction with Acids
Metals react with acids to form metallic salts and hydrogen. Example: Zinc reacts with dilute hydrochloric acid to form zinc chloride and hydrogen.
Zn + 2HCl → ZnCl₂ + H₂
Most non-metals do not react with acids. Some non-metals like sulphur reacts with concentrated nitric acid to form sulphur dioxide, nitrogen dioxide and water.
S + 4HNO₃ → SO₂ + 4NO₂ + 2H₂O
• Reaction with Bases
In general, metals do not react with bases. Few metals like zinc, aluminium reacts with bases to form compounds and hydrogen gas.
Example: Zinc reacts with sodium hydroxide to form sodium zincate and hydrogen.
Zn + NaOH → Na₂ZnO₂ + H₂
• Metals replace metals
A more reactive metal replaces a less reactive metal from its salt solution.
Example: Magnesium replaces copper from copper sulphate solution to form magnesium sulphate and copper.
Mg + CuSO₄ → MgSO₄ + Cu
Zinc replaces copper from copper solution to form zinc sulphate and copper.
Zn + CuSO₄ → ZnSO₄ + Cu
Iron replaces copper from copper sulphate solution to form iron sulphate and copper.
Fe + CuSO₄ → FeSO₄ + Cu
Based on the reactivity of metals, they can be arranged in decreasing order of their activity.
5. Activity series of metals
The arranging of metals in the decreasing order of their reactivity is called activity series of metals.
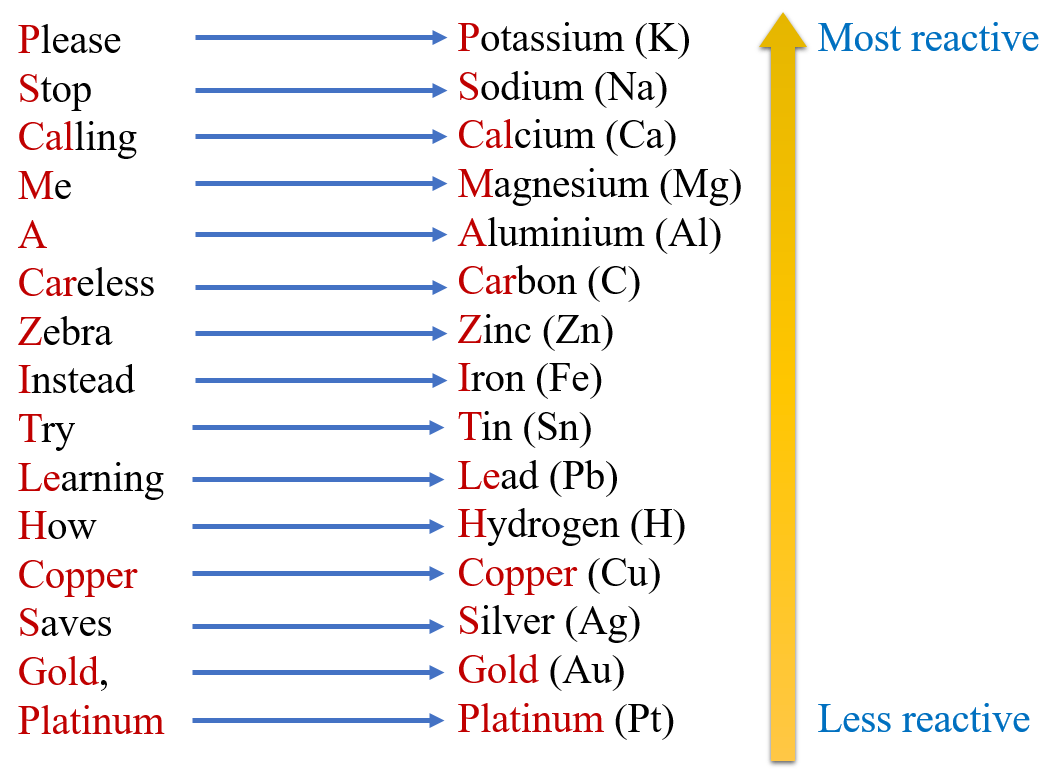
Potassium, Sodium, Calcium, Magnesium, Aluminium, Carbon, Zinc, Iron, Tin, Lead are more reactive than Hydrogen.
Copper, Silver, Gold, Platinum are less reactive than Hydrogen.
6. Zinc and aluminium react with both acids and bases and therefore are called amphoteric metals.
7. Noble metals
• Metals like gold, silver, platinum etc. retain their lustre because they do not react with air, water or acids. So they are called noble metals.
• Gold dissolves in aqua regia. Aqua regia is a mixture of concentrated nitric acid and concentrated hydrochloric acid in the ratio 1:3.
• Pure gold is 24 karat. It is very soft and cannot be used for making ornaments. So it is mixed with some silver or copper to make it hard.
8. Uses of Metals
• Iron is used for making pins, nails, tools, machines, construction of buildings, bridges etc.
• Aluminium is used for making utensils, wires, parts of aircraft, vehicles, machines, for packing food and medicines etc.
• Copper is used for making wires, vessels, electric gadgets etc.
• Gold is used for making jewellery, coins etc.
• Silver is used for making jewellery, coins etc.
• Platinum is used for making jewellery, plugs in vehicles etc.
• Sodium compounds are used as common salt, chemicals etc.
• Calcium compounds are used for making cement, glass etc.
9. Uses of Non-metals
• Sulphur is used for making sulphuric acid, salts of metals etc.
• Oxygen is used for respiration by living things, burning of fuels etc.
• Nitrogen is used for making ammonia which is used for making fertilizers.
• Hydrogen is used for making ammonia which is used for making fertilizers, as fuel in rockets, for welding etc.
• Chlorine is used to kill germs in water.
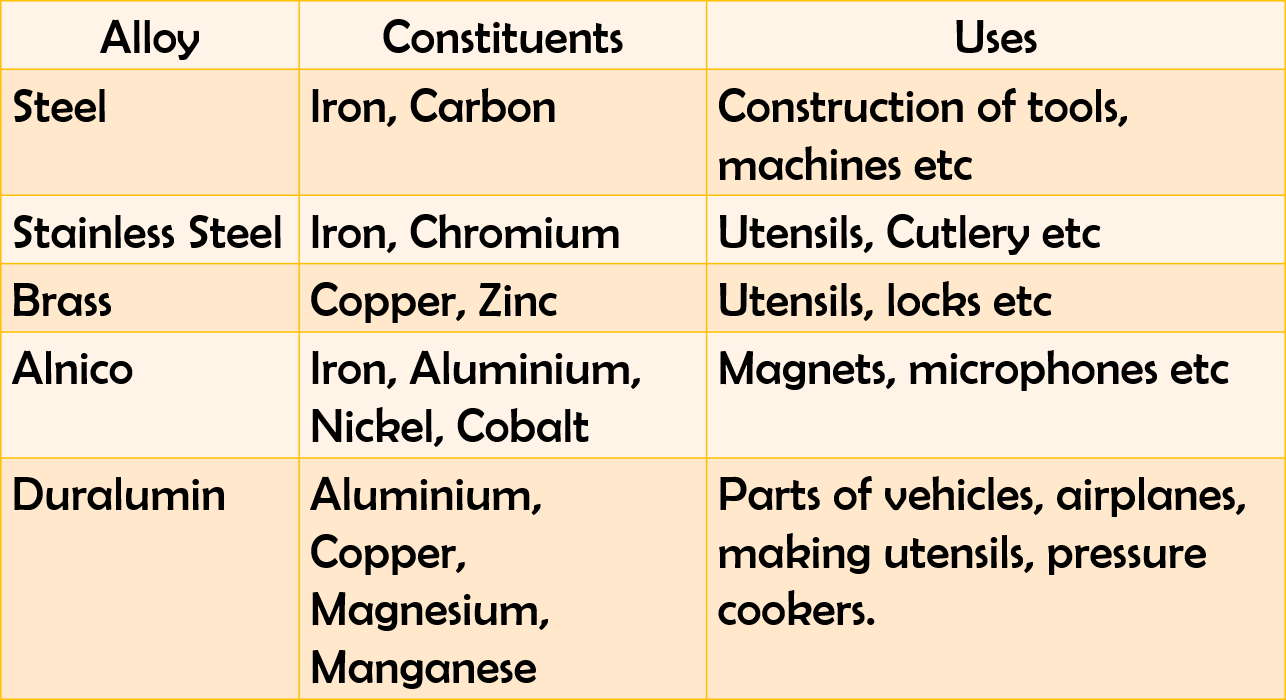
10. Alloys
An alloy is a homogeneous mixture of a metal with other metals or non-metal.
11. What is Corrosion? Ways to prevent it.
The surface of some metals gets corroded when exposed to moist air for a long time. This is called corrosion. It can be prevented by following ways:
• Applying oil/grease.
• Applying paint
• Galvanisation (The process of depositing a layer of a metal like chromium or zinc on iron or steel.)
• Electroplating (The process of depositing a layer of any desired metal on another material by the means of electricity)
• Alloying (An alloy is a mixture of a metal with one or other elements)
12. Exceptions
• Electrical Conductivity: Carbon (in the form of graphite) is a non-metal which conducts electricity like other metals.
• Lustre: Iodine is a non-metal which is lustrous, having a shining surface like other metals.
• Hardness and softness: Alkali metals like lithium, sodium and potassium are soft like non-metals. Carbon (in the form of diamond) is a non-metal which is extremely hard like other metals.
• Physical state: Mercury is a metal which is at liquid state at room temperature.
• Melting and boiling points: Sodium, potassium, cesium and gallium are metals that have low melting points. Diamond is a non-metal which has a very high melting and boiling point.
• Density: Alkali metals like lithium, sodium and potassium have low densities like non-metals.
13. Malleability
The property of metals by which they can be beaten into thin sheets is called malleability.
14. Ductility
The property of metal by which it can be drawn into wires is called ductility.
15. Melting and Boiling Points
• The melting point is defined as the point at which materials changes from a solid to a liquid.
• The boiling point of a liquid is the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure of the liquid’s environment. At this temperature, the liquid is converted into a vapour.
16. CuSO₄ + ZnSO₄ = no reaction because copper is less reactive than zinc sulphate.
17. Common acids and bases
Acetic acid [CH₃COOH], Hydrochloric acid [HCl], Nitric acid [HNO₃], Sulphuric acid [H₂SO₄] are commonly used acids.
Sodium hydroxide [NaOH], Potassium hydroxide [KOH], Calcium hydroxide [Ca(OH)₂], Ammonium hydroxide (NH₄OH) are the commonly used bases.
18. Allotropes
The term allotropes refers to one or more physical forms of a chemical element that occurs in the same physical state. Allotropes may show differences in chemical & physical properties.
Examples of allotropes:
• Diamond and graphite are the allotropic forms of carbon.
• Red phosphorus & white phosphorus are allotropes of phosphorus.
• Rhombic sulphur, monoclinic sulphur & plastic sulphur are the allotropic forms of sulphur.
19.

20. Sodium metal is very reactive. It reacts with oxygen and water. A lot of heat is generated in the reaction. Therefore, it is stored in kerosene.
21. Magnesium is found in plants. In what form it is found in them?
Magnesium is very essential in photosynthesis in plants. Without magnesium, chlorophyll cannot capture sun energy needed for photosynthesis. Magnesium is required to give leaves their green colour. Magnesium in plants is located in the enzymes, in the heart of the chlorophyll molecule.
23. In a chemical reaction, new substances are formed. These substances are different from those which underwent the reaction.
24. If a substance cannot be broken down further by chemical reactions, by cooling, heating, or by electrolysis, it is called ‘element’. Sulphur is an element. So is iron. Carbon, too, is an element. The smallest unit of an element is atom. A sample of an element contains only one kind of atom. The atom of an element remains unaffected by physical changes in the element. Example: An atom of liquid sulphur would be exactly the same as the atom of solid or vapour sulphur.
25. Double Displacement Reaction
In this reaction, exchange of ions occurs between two compounds. It is also called precipitation reaction because in this type of reaction, a precipitate is also formed.
Example:
AgNO₃ + NaCl → AgCl + NaNO₃
In the above reaction, AgNO₃ (silver nitrate) reacts with NaCl (sodium chloride) to form AgCl (silver chloride), which is the precipitate, and NaNO₃ (sodium nitrate).
26. On the basis of composition, alloys are of two types:
• Substitutional alloys in which atoms of one element randomly replace the atoms of another metal.
• Interstitial alloys in which small atoms like hydrogen, boron, carbon and nitrogen occupy the holes in the crystal structure of the metal.
On the basis of constituent elements, alloys are of two types:
→ Ferrous alloys: They contain iron as base metal. Example: steel, alnico etc.
→ Non-Ferrous alloys: They do not contain iron as base metal. Example: brass, bronze, duralumin etc.
No comments:
Post a Comment