Chapter 6 - Combustion and Flame Notes
1. What is combustion?
A chemical process in which a substance reacts with oxygen to give off heat is called combustion. The substance that undergoes combustion is said to be combustible. It is also called a fuel. The fuel may be solid, liquid or gas. Sometimes, light is also given off during combustion, either as a flame or as a glow.
Example: In the burning of magnesium, the magnesium is the combustible substance.
2. What are combustible and non-combustible substance?
These are the substances which burn in air to produce heat or sometimes light are called combustible substances.
Example: Wood, LPG, CNG etc.
These are the substances which don’t burn in air to produce heat are called non-combustible substances.
3. Activity to prove that air is necessary for burning
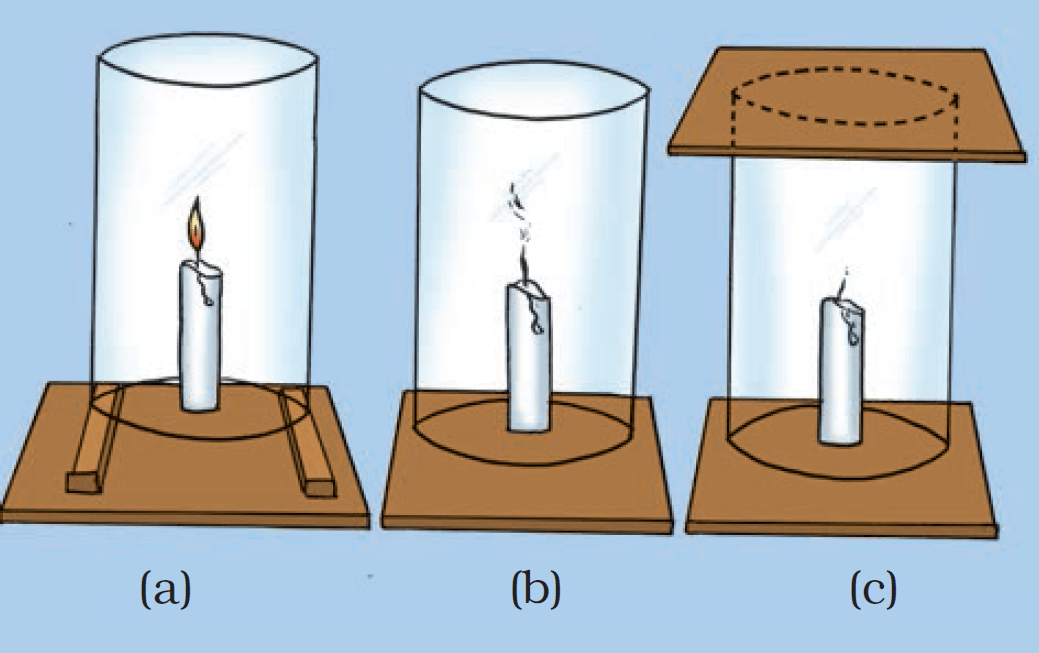
Fix a lighted candle on a table. Put a glass chimney over the candle and rest it on a few wooden blocks in such a way that air can enter the chimney. Observe what happens to the flame. Now remove the blocks and let the chimney rest on the table. Again observe the flame. Finally, put a glass plate over the chimney. Watch the flame again. We find that for combustion, air is necessary. The candle burns freely in case (a) when air can enter the chimney from below. In case (b), when air does not enter the chimney from below, the flame flickers and produces smoke. In case (c), the flame finally goes off because the air is not available.
4. Ignition temperature
The lowest temperature at which a substance catches fire is called its ignition temperature.
5. A combustible substance cannot catch fire or burn as long as its temperature is lower than its ignition temperature. Kerosene oil and wood do not catch fire on their own at room temperature. But, if kerosene oil is heated a little, it will catch fire. But if wood is heated a little, it would still not catch fire. It is essential for a substance to reach ignition temperature to burn.
6. The substances which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances. Examples of inflammable substances are petrol, alcohol, Liquified Petroleum Gas (LPG) etc.
7. How do we control fire?
Water cools the combustible material so that its temperature is brought below its ignition temperature. This prevents the fire from spreading. Water vapour s also surround the combustible material, helping in cutting off the supply of air. So, the fire is extinguished.
8. Three essential requirements for producing fire are fuel, air (to supply oxygen) and heat (to raise the temperature of the fuel beyond the ignition temperature). Fire can be controlled by removing one or more of these requirements. The job of a fire extinguisher is to cut off the supply of air, or to bring down the temperature of the fuel, or both.
9. Types of combustion
• Rapid combustion
Rapid combustion requires external heat energy to start. This reaction results in enormous amounts of light and heat energy. This type of combustion occurs rapidly. There are substances like phosphorus which burn in air at room temperature.
• Spontaneous combustion
Spontaneous combustion doesn’t require any external energy to start the combustion process. This combustion starts spontaneously at room temperature itself due to self-heating.
• Explosion combustion
Explosion combustion happens very rapidly releasing enormous amounts of heat, light, and sound energy. This combustion process is simply can be said as an explosion.
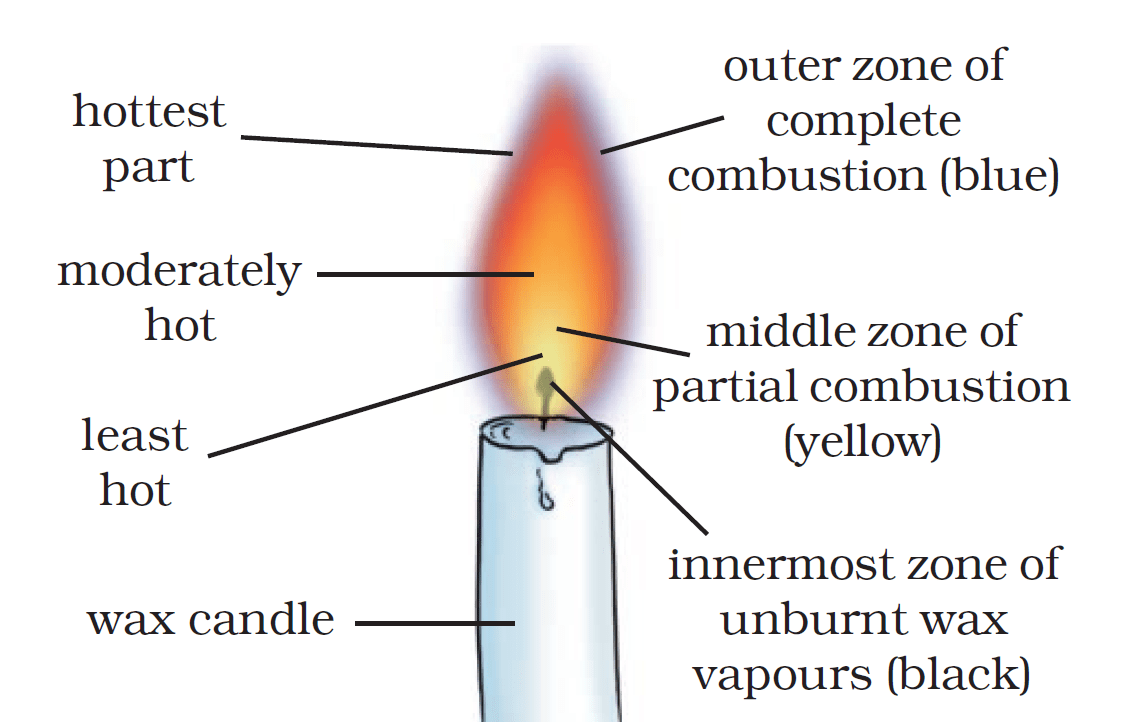
9. Structure of a Flame
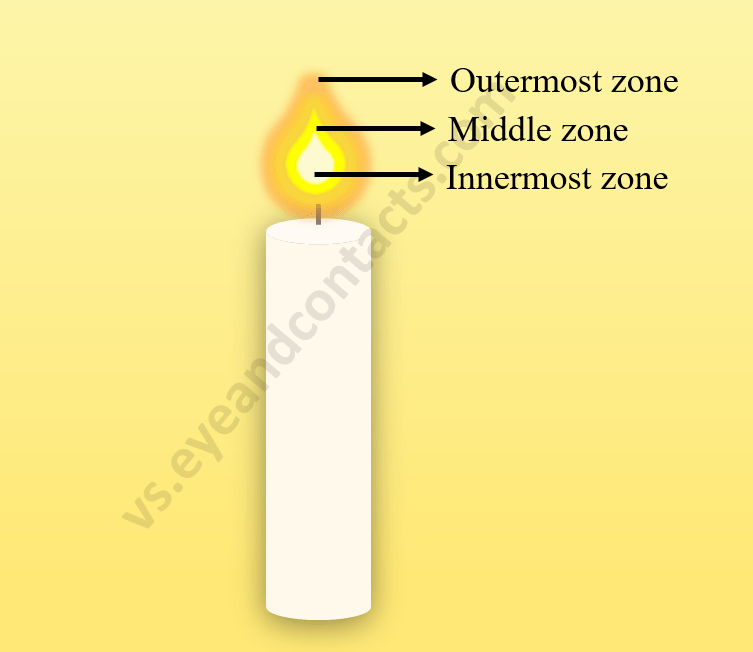
The substances which vapourise during burning, give flames. Example: Kerosene oil and molten wax rise through the wick and are vapourised during burning and form flames. Charcoal, on the other hand, does not vapourise and so does not produce a flame. Goldsmiths blow the outermost zone of a flame with a metallic blow-pipe for melting gold and silver.
10. What is a fuel?
Fuels are substances that produce heat and light energy on burning. Example: Wood, Petrol, Kerosene etc. A good fuel is one which is readily available. It is cheap. It burns easily in air at a moderate rate. It produces a large amount of heat. It does not leave behind any undersirable substances.
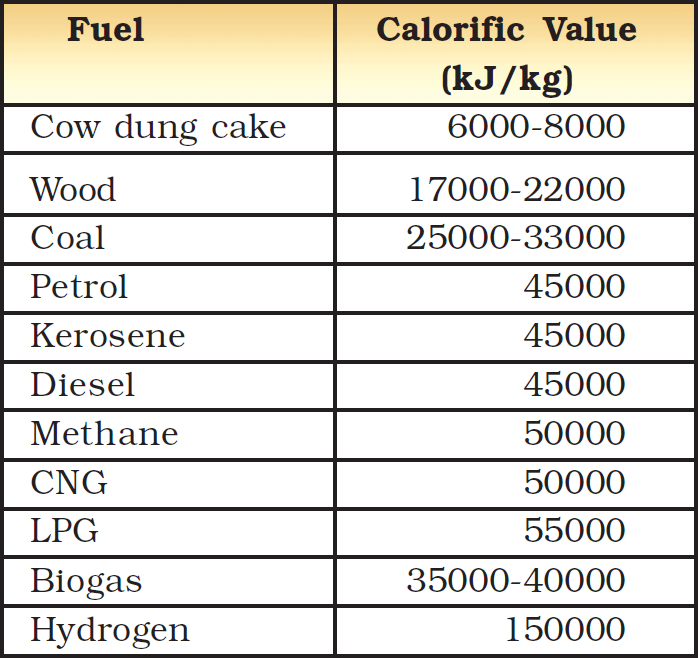
11. Fuel efficiency
The amount of heat energy produced on complete combustion of 1 kg of a fuel is called its calorific value. The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg).
12. Burning of Fuels Leads to Harmful Products
The increasing fuel consumption has harmful effects on the environment.
• Carbon fuels like wood, coal, petroleum release unburnt carbon particles. These fine particles are dangerous pollutants causing respiratory diseases, such as asthma.
• Incomplete combustion of these fuels gives carbon monoxide gas. It is a very poisonous gas. It is dangerous to burn coal in a closed room. The carbon monoxide gas produced can kill persons sleeping in that room.
• Combustion of most fuels releases carbon dioxide in the environment. Increased concentration of carbon dioxide in the air is believed to cause global warming.
• Burning of coal and diesel releases sulphur dioxide gas. It is an extremely suffocating and corrosive gas. Moreover, petrol engines give off gaseous oxides of nitrogen. Oxides of sulphur and nitrogen dissolve in rain water and form acids. Such rain is called acid rain. It is very harmful for crops, buildings and soil. The use of diesel and petrol as fuels in automobiles is being replaced by CNG (Compressed Natural Gas), because CNG produces the harmful products in very small amounts. CNG is a cleaner fuel.
13. In the sun, heat and light are produced by nuclear reactions.
14. History of the matchsticks
More than five thousand years ago small pieces of pinewood dipped in sulphur were used as matches in ancient Egypt. The modern safety match was developed only about two hundred years ago. A mixture of antimony trisulphide, potassium chlorate and white phosphorus with some glue and starch was applied on the head of a match made of suitable wood. When struck against a rough surface, white phosphorus got ignited due to the heat of friction. This started the combustion of the match. However, white phosphorus proved to be dangerous both for the workers involved in the manufacturing of matches and for the users. These days the head of the safety match contains only antimony trisulphide and potassium chlorate. The rubbing surface has powdered glass and a little red phosphorus (which is much less dangerous). When the match is struck against the rubbing surface, some red phosphorus gets converted into white phosphorus. This immediately reacts with potassium chlorate in the matchstick head to produce enough heat to ignite antimony trisulphide and start the combustion.
15. Fire extinguisher
The most common fire extinguisher is water. But water works only when things like wood and paper are on fire. If electrical equipment is on fire, water may conduct electricity and harm those trying to douse the fire. Water is also not suitable for fires involving oil and petrol. Since, water is heavier than oil, it sinks below the oil, and oil keeps burning on the top. For fires involving electrical equipment and inflammable materials like petrol, carbon dioxide is the best extinguisher. CO₂, being heavier than oxygen, covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled. The added advantage of CO₂ is that in most cases it does not harm the electrical equipment. CO₂ can be stored at high pressure as a liquid in cylinders. When released from the cylinder, CO₂ expands enormously in volume and cools down. So, it not only forms a blanket around the fire, it also brings down the temperature of the fuel. That is why it is an excellent fire extinguisher. Another way to get CO₂ is to release a lot of dry powder of chemicals like sodium bicarbonate (baking soda) or potassium bicarbonate. Near the fire, these chemicals give off CO₂.
16. Earlier wood was used as domestic and industrial fuel. But now it has been replaced by coal and other fuels like LPG. In many rural parts of India, people still use wood as a fuel because of its easy availability and low cost. However, burning of wood gives a lot of smoke which is very harmful for human beings. It causes respiratory problem. Also, trees provide us with useful substances which are lost when wood is used as fuel. Moreover cutting of trees leads to deforestation which is quite harmful to the environment.
17. Global warming is the rise in temperature of the atmosphere of the earth. This results, among other things, in the melting of polar glaciers, which leads to a rise in the sea level, causing floods in the coastal areas. Low lying coastal areas may even be permanently submerged under water.
No comments:
Post a Comment